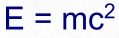Introduction



Advertisement
You and I are solar powered, at least indirectly. It’s the Sun's energy that grows plants that (one way or another) we eat
and get our energy from. Until Einstein derived E = mc
2
from his Special Theory of Relativity it was a complete mystery
as to what the fuel source of the Sun was. For example, it had been calculated that if the Sun was made of coal it would
use up its total fuel supply in about 6000 years. With an ever-increasing understanding of the age of the Solar System,
based mainly on geological research, it was clear that there must be a process going on within the Sun that we didn't
understand. We now know that the Sun is about 4.6 billion years old and has about the same length of time before it will
have used up all of its fuel. This page deals with nuclear fusion, and in doing so explains why the Sun can produce so
much energy over such a long period of time.

The Sun
We are all solar powered

Fusion - An Atomic Process
We have seen in previous pages in this series that a heavy atom, such as uranium, can "fall apart", that is, undergo
fission. When this happens, a little of the mass of the original atom is turned into energy. It's also possible to turn mass
into energy by taking less massive atoms, such as hydrogen, and squeezing them together to form another type of, and
heavier, atom. This process is called nuclear fusion.
Fusion can occur with many different kinds of atom. In fact, over a third of all the different kinds of atoms, when fused,
release energy. This is a point we will return to later, but for now we will concentrate on the simplest form of nuclear
fusion, that of hydrogen.
Hydrogen is the simplest of all atoms. The first isotope of hydrogen contains nothing but a single proton, with a single
electron in "orbit" around it. If the hydrogen atom is given energy (for example, by heating it or speeding it up) the
electron is "shaken" away and we are left with just a proton. Strictly speaking we should now call the "atom" an "ion", but
in this page we will continue to call it an atom in order to keep things simple (with apologies to chemists).
A proton (i.e. the nucleus of a hydrogen "atom") has a positive electrical charge, that is, it acts like the positive end of a
magnet. If we bring two protons together they repel each other. The closer we try to push the protons together the more
energy we need to overcome the repulsion. You may have experienced something similar yourself. If not, find two
magnets and try to push either both the negative or both the positive ends together. You will find that when the magnets
are far apart they are easy to move towards each other, but as they get closer more and more energy is required in order
to push them together:
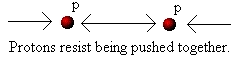
If we apply a lot of energy (on an atomic scale) we overcome the magnetic resistance and the two protons stick together;
they have fused. In doing so they give up a little of their mass in the form of energy. In fact, the energy released is
greater than the energy that was required to force the two protons together. We now have a source of energy: nuclear
fusion.
Why is energy released during the fusion of hydrogen? We said that when two protons are forced together they fuse.
However, that's not all that happens. What actually happens is that one of the protons changes into another particle; it
"transmutes" from being a proton into being a neutron. Not only that, but in doing so it ejects two further particles: a
positron (a positively charged electron) and a strange, almost mass-less, particle called a neutrino. We can show this in a
schematic diagram:
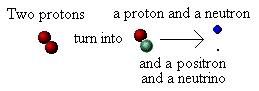
We now have the nucleus of an atom that is the second isotope of hydrogen, called deuterium (d). It contains one proton
and one neutron. The positron and neutrino go flying off with kinetic energy supplied by converting some of the mass of
the transmuted proton into kinetic energy, in accordance with E = mc
2
.
The Sun's Fusion
When the Sun was formed about 4.6 billion years ago, it did so out of a huge cloud of gas. Most of that gas was
hydrogen, but it also contained some helium (about 30%) and small amounts of many other elements such as carbon,
oxygen, silicon, and so on. The gas cloud contracted under its own gravity and started to spin, in doing so ejecting most
of the heavier elements, some of which became the planets, asteroids and comets, and some of which eventually ended
up as you and me. What remained was a huge ball of mostly hydrogen and helium that we now call the Sun.
Deuterium
The gas in the Sun continued to contract under its own gravity until the pressure at the core grew to enormous
proportions (about 100 billion times the pressure of air on the Earth). A law of nature states that if you squeeze anything
it heats up. As the centre of the Sun became more and more compressed the temperature at the core reached about 15
million degrees Celsius (27 million degrees Fahrenheit). This meant that the protons (hydrogen "atoms") at the core
possessed, in atomic terms, huge amounts of energy. So much energy in fact, that some of them could overcome any
magnetic resistance and fuse into deuterium, releasing even more energy in the process. This is the first stage in the
Sun's source of energy:
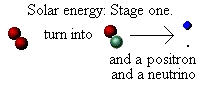
Further energy is provided by the positron. This is a form of antimatter. It has exactly the same properties as an electron
(in terms of mass etc.), but has an opposite, and equally strong, electrical charge. The Sun's core contains many "free"
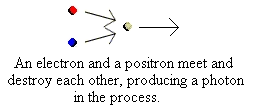
electrons in what is called a "plasma" (a sort of high energy gas). The positron soon meets an electron and when it does
so the two annihilate each other producing a high-energy photon, i.e. "light". Our star is shining, but not yet in the visible
part of the spectrum:
Helium-3
The second major stage is the formation of helium-3 (He-3), again by a process of fusion. There are four isotopes of
helium, He-3, -4, -5, and -6. The latter two isotopes, He-5 and -6, have short half-lives (in the case of He-5 only 6 x
10
–20
seconds!) and we will not be concerned with them here. Each helium atom, by definition, has two protons.
So far we have an "atom" of deuterium (a proton and neutron) in a sea of highly energetic hydrogen "atoms" (protons).
Sooner or later (but usually sooner!) the two types of atoms will collide and fuse. When they do so they combine to
make the atomic nucleus of helium-3, and eject yet another high-energy photon:
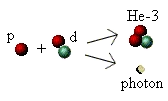
Helium-4
Lastly, the third stage is the production of helium-4. In this process, two He-3 "atoms" come together and fuse,
releasing two protons. The two protons fly off in different directions and go back to being hydrogen "atoms", from which
they can take part in the whole process again. The process looks like this:
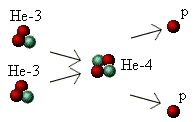
Throughout each stage some mass was converted into energy and a total of 6 high-energy photons was produced.
However, we still haven't seen any visible light. The visible light from the Sun is due to the ejected particles jostling
with other atoms and so transferring some energy to them. This causes the latter atoms to emit photons with a wide
range of frequencies, including those in the visible part of the spectrum. At last, we have visible sunlight!
The three stages outlined here are often taken together and called the "proton-proton" chain.
The Total Energy
The total energy released in all three stages is around 4 x 10
–12
J (25 MeV). This is less than is produced during in a
single uranium-235 fission process. However, if we take the total number of particles into account in each process, we
find that we get around 7 times more energy per particle in hydrogen fusion than we get from uranium-235 fission. In
other words, it is a much more efficient process. Even so, only around 0.7% of the mass of the Sun's protons
eventually ends up as light.
The energies we have been talking about, while large on an atomic scale, are still very small in everyday terms.
However, there is something we must take into account about the Sun, and that is it's enormous! It has the same
volume as 1.3 million planet Earths. Some numbers about the rate at which nuclear fusion takes place in the Sun will
be instructive:
There are around 8.5 x 10
37
fusion cycles per second at the Sun's core.
This leads to a total energy output from the Sun of around 3.8 x 10
26
joules per second.
The Sun converts 4 million tonnes (4.4 million tons) of mass into energy every second.
Each square centimetre of the Sun's surface is as bright as a 6000 W light bulb.




The amount of mass converted into energy at the Sun's core is stunning. However, the Sun has a mass of around 2 x
10
30
kg and has enough hydrogen to continue its proton-proton chain for around another 4 billion years. After that, as
we shall see, the Sun will use another process to keep shining for a "little" longer.
Lastly, it's worth mentioning that the "sunlight" produced at the core doesn't just fly off into space. As has been seen, the
proton-proton chain doesn't even produce light in the visible part of the spectrum. Instead, the high-energy protons and
resultant kinetic energy produced induces other atoms in the Sun to vibrate and, in turn, release photons of many
different frequencies, including those in the visible part of the spectrum. These photons are re-absorbed and re-emitted
by adjacent atoms, each, on average, slightly closer to the surface of the Sun. Finally, after around 100 to 200 thousand
years an atom at the surface of the Sun absorbs and then re-emits a photon, which flies off into space. Then, if heading
in our direction, it takes around 8.5 minutes for the photon to reach the Earth. All of the daylight that we use to see by
started on its journey a very long time ago.

(The dark blotches are sunspots – magnetic storms on the
Sun’s surface, many of them much bigger than the Earth.)
The Sun: Turning mass into energy.
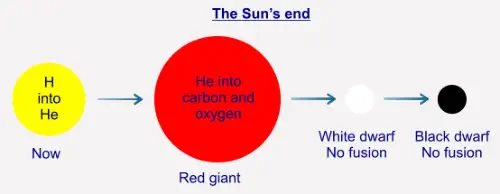
Fusion in other atoms - the Sun's End
Fusion is possible in atoms other than hydrogen and helium. In fact, fusion, one way or another, is possible with almost
any kind of atom. In nature the heaviest atoms that we could encounter are of uranium, but scientists have been able to
"build" heavier atoms in atomic accelerators, such as the one at CERN. This is done by firing atoms at each other until
they "stick" (fuse) together. Most of the resultant atoms have very short half-lives. Another feature of such fusion is that it
takes a lot of energy while little, or even none, is given back out.
It turns out that the atom that gives up the most of its mass in the form of energy during fusion is the humble hydrogen
atom, i.e. the lightest atom of them all. As we progressively fuse heavier and heavier atoms we get progressively less and
less energy back out. In fact, we get at least some energy back out all the way up to the element containing 26 protons.
That element is iron (Fe). For iron, and all heavier atoms, we need to put more energy in than we get back out in order to
achieve fusion. This is called an endothermic process.
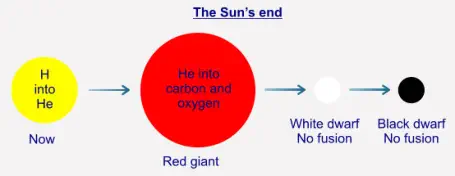
As we have seen, the Sun's fusion is exothermic, that is, more energy is "produced" than was entered into the system.
We have also seen that the Sun is slowly converting its hydrogen into helium-4. Once the Sun has used up all of its
hydrogen in this process its core will contract because there is now not enough radiation ("light" and so on) being
produced to withstand the gravitational pressure from the outer layers. Although the core contracts, the very outer layers
of the Sun will expand, possibly out to the orbit of Mars (the Earth will be turned into a wisp of gas in the process). The
Sun will then be what is a called a "red giant". The contraction of the core raises its pressure, and by doing so also raises
its temperature. This results in there being enough energy for the He-4 to begin to fuse into (mostly) carbon and oxygen,
and so produces enough radiation energy ("light") to stabilise the star. Once the He-4 has been used up no further fusion
will take place at the core, and the Sun will contract again into a state called a "white dwarf". At that point, the Sun will no
longer be active as such, and will just continue to cool over many millions of years, eventually becoming a cold "black
dwarf":

Supernova
Apart from the fact that we live near it, there isn’t anything particularly special about the Sun. It's just an average star.
Some stars are much heavier than the Sun, and these go through further fusion chains, producing even heavier
elements. A detailed description of all the phases in a very massive star's life is beyond the scope of this page, but we
will look briefly at what happens to a star with a mass greater than about four times that of the Sun.
Such massive stars start by converting hydrogen into helium, then, when the hydrogen is used up, helium into carbon
and oxygen, just as in the Sun. However, the core of such a star is so massive that when the helium has been used up it
gravitationally contracts still further, and produces yet heavier elements via the fusion process. These contractions
continue until the star starts to fuse its remaining elements into iron.
As we have noted, fusion into iron is endothermic, i.e. it takes more energy to do than we get back out. For the star there
is now no radiation (of any significance) being produced to stop the outer layers from gravitationally collapsing onto the
core. Within a few seconds of the star's core starting to fuse into iron, the outer layers start accelerating inwards at
tremendous speeds. As they do so they become ever more dense as more and more material is compressed together
and accelerated. The highly compressed material eventually reaches near-light speed (in only a matter of seconds),
before it crashes down onto the dense core of the star. When this happens the core is compressed slightly, then bounces
back with such enormous force that it blows the outer layers back out into space. The star has, effectively, blown itself
apart in what is called a supernova explosion. The energy released in a single supernova is more than many billions of
atom bombs being detonated all at once.
The picture below shows the remnants of a supernova. This was a "nearby" (168,000 light years away) supernova that
was recorded in 1987, and given the name SN1987A. The ring of material that is expanding away from the central core is
actually a bubble of gas, but we are looking at it edge on:

The energy released in the explosion is such that many different types of atoms in the expanding gas shell are fused
together to form all the elements heavier than iron; such as copper, gold and uranium. Next time you see some gold try to
remember that it was created billions of years ago by a process of fusion during the last moments of a massive star's
active life!
Generally, what is left of the star is a rapidly expanding bubble of gas rushing off into space, and a super-dense central
core composed almost entirely of neutrons. The pressure of the outer layers crashing down onto the central core during
the initial implosion causes the atomic protons and free electrons in the core to fuse into neutrons. The star is now called
a neutron star. From this point on, the bare core that the star has become starts to cool, emitting energy into space as it
does so.
For even more massive stars the end result is a black hole. These stars have so much mass that as the outer layers hit
the core they compress it into such a state that even the resulting neutrons are compressed into other particles, possibly,
for a very short time, the building blocks of neutrons and protons, called quarks. This results in such an enormous mass
being squeezed down into such a very small size that, under the pressure of its own gravity, the core continues to
collapse until it becomes "infinitely small". The gravitational pull of such an object is so strong that not even light can
move fast enough to escape it, and we have a "black hole".

The Crab Nebula
The supernova that created the Crab Nebula was seen on Earth in AD1054.
What remains is an expanding gas cloud and a central neutron star.

H-bombs
The process of hydrogen fusion that is the source of energy for stars is the same as is used in H- (hydrogen) bombs.
Briefly, in an H-bomb, a small amount of hydrogen (usually the isotopes deuterium and tritium) is compressed together to
the point at which it fuses into helium, releasing enormous amounts of energy. It takes a huge amount of pressure to
compress hydrogen to the point at which it fuses, and in order to make such a bomb light enough to be delivered by an
aircraft or missile, the hydrogen compressing "agent" is actually an atom bomb surrounding the hydrogen.
Nuclear (atomic and hydrogen) weapons often have their "yields" (explosive power) measured as the equivalent of the
energy released in a quantity of the explosive TNT, measured in tons. An atom bomb's power is typically measured in
kilotons, i.e. thousands of tons. The two atomic bombs dropped on Japan and the end of World War II were in the 15 to
20 kiloton range. The more powerful H-bombs, however, have their yields typically measured in megatons, i.e. millions of
tons.
On detonation, an H-bomb produces He-3 and He-4 in the same way as the Sun. While these two elements are harmless
in themselves, the atoms move at such high speeds that they can damage anything around them. They can also strike
other, heavier, atoms, breaking them down into harmful radioactive elements. And, of course, there is the radiation
caused by the triggering atom bomb. For these reasons, nuclear weapons tests were eventually carried out under
ground, where most of the radiation could be contained. Even so, each detonation caused environmental damage and
still released a quantity of harmful radiation from the atom bomb component.

Nuclear Power from Fusion
As described in another page in this series, nuclear power stations use nuclear fission (the breaking apart of heavy
elements) as their source of power. This has a number of disadvantages (but not any more than conventional power
stations, depending on your point of view). For example, using fission as a power source produces radiation, and
although it is mostly contained there is the problem of both decommissioning old nuclear power stations and the question
of what to do with the contaminated waste products. Allied to that, as with conventional power stations, there is only so
much of the raw materials available from which to produce the power.
Great advances have been have made in using other, renewable sources of power, such as energy derived from wind,
wave and solar sources. However, because of the limited total renewable energy incident on the Earth, these sources of
power will never be enough to meet all of the world's rapidly growing demands, and they are often expensive ways of
producing usable power in the first place. All in all, it would be good if we could find a cheap, safe and abundant source
of energy. So why not use controlled fusion?
The answer is simple: we don't know how to, at least not yet. Small scale fusion has been carried out for many years in
experimental situations, but controllable, large scale hydrogen fusion has yet to be achieved.
There are three points to note about controlled hydrogen fusion:
We have an almost inexhaustible supply of the raw materials, in sea water:
We can easily, and safely, extract the hydrogen in sea water. Using the fusion process
there is enough energy in about a gallon (about 4 litres) of water to supply America's
energy requirements for a whole day. The whole world would only need a few of
buckets of water a day.
It's safe:
The end products of hydrogen fusion are helium-3 and helium-4. Helium in itself is non-
radioactive and non-reactive chemically and is therefore very safe (we even fill
children's balloons with it). In addition, there’s no danger of a meltdown – the process
just stops if there is insufficient energy in the system.
At the moment, it is very, very expensive:
Controlled hydrogen fusion has been achieved in very large machines such as particle
accelerators. Hydrogen is put into the system and accelerated or heated thereby fusing
into helium, but in each case the system used is experimental and very expensive.



The goal is to be able to find a way of making the process practical and cheap. This is thought to be so important that
every major industrial nation on the planet has access to accelerators, with the hope of finding a way of making the
fusion process work efficiently. There isn't even any guarantee that it will work, but if it does we will have solved all of our
energy problems both now and a long way into the future. To this end a number of countries are building experimental
fusion reactors of competing designs, often with much collaboration between nations and scientists. However, the task is
so difficult and complex that even if successful it will still be decades before we see the first commercial scale power
plants come into operation.
If we do ever solve the problem of controlled nuclear fusion, at its root will be the conversion of mass into energy, in
accordance with this little equation:
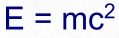
Advertisement
Nuclear fusion
Introduction



Advertisement

You and I are solar powered, at least indirectly. It’s the Sun's
energy that grows plants that (one way or another) we eat and
get our energy from. Until Einstein derived E = mc
2
from his
Special Theory of Relativity it was a complete mystery as to
what the fuel source of the Sun was. For example, it had been
calculated that if the Sun was made of coal it would use up its
total fuel supply in about 6000 years. With an ever-increasing
understanding of the age of the Solar System, based mainly on
geological research, it was clear that there must be a process
going on within the Sun that we didn't understand. We now
know that the Sun is about 4.6 billion years old and has about
the same length of time before it will have used up all of its fuel.
This page deals with nuclear fusion, and in doing so explains
why the Sun can produce so much energy over such a long
period of time.

The Sun
We are all solar powered
Fusion - An Atomic Process
We have seen in previous pages in this series that a heavy
atom, such as uranium, can "fall apart", that is, undergo fission.
When this happens, a little of the mass of the original atom is
turned into energy. It's also possible to turn mass into energy by
taking less massive atoms, such as hydrogen, and squeezing
them together to form another type of, and heavier, atom. This
process is called nuclear fusion.
Fusion can occur with many different kinds of atom. In fact, over
a third of all the different kinds of atoms, when fused, release
energy. This is a point we will return to later, but for now we will
concentrate on the simplest form of nuclear fusion, that of
hydrogen.
Hydrogen is the simplest of all atoms. The first isotope of
hydrogen contains nothing but a single proton, with a single
electron in "orbit" around it. If the hydrogen atom is given energy
(for example, by heating it or speeding it up) the electron is
"shaken" away and we are left with just a proton. Strictly
speaking we should now call the "atom" an "ion", but in this page
we will continue to call it an atom in order to keep things simple
(with apologies to chemists).
A proton (i.e. the nucleus of a hydrogen "atom") has a positive
electrical charge, that is, it acts like the positive end of a magnet.
If we bring two protons together they repel each other. The
closer we try to push the protons together the more energy we
need to overcome the repulsion. You may have experienced
something similar yourself. If not, find two magnets and try to
push either both the negative or both the positive ends together.
You will find that when the magnets are far apart they are easy
to move towards each other, but as they get closer more and
more energy is required in order to push them together:
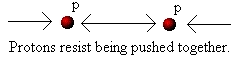
If we apply a lot of energy (on an atomic scale) we overcome
the magnetic resistance and the two protons stick together; they
have fused. In doing so they give up a little of their mass in the
form of energy. In fact, the energy released is greater than the
energy that was required to force the two protons together. We
now have a source of energy: nuclear fusion.
Why is energy released during the fusion of hydrogen? We said
that when two protons are forced together they fuse. However,
that's not all that happens. What actually happens is that one of
the protons changes into another particle; it "transmutes" from
being a proton into being a neutron. Not only that, but in doing
so it ejects two further particles: a positron (a positively charged
electron) and a strange, almost mass-less, particle called a
neutrino. We can show this in a schematic diagram:
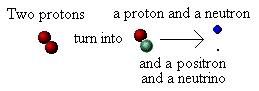
We now have the nucleus of an atom that is the second isotope
of hydrogen, called deuterium (d). It contains one proton and one
neutron. The positron and neutrino go flying off with kinetic
energy supplied by converting some of the mass of the
transmuted proton into kinetic energy, in accordance with E =
mc
2
.

The Sun's Fusion
When the Sun was formed about 4.6 billion years ago, it did so
out of a huge cloud of gas. Most of that gas was hydrogen, but it
also contained some helium (about 30%) and small amounts of
many other elements such as carbon, oxygen, silicon, and so on.
The gas cloud contracted under its own gravity and started to
spin, in doing so ejecting most of the heavier elements, some of
which became the planets, asteroids and comets, and some of
which eventually ended up as you and me. What remained was
a huge ball of mostly hydrogen and helium that we now call the
Sun.
Deuterium
The gas in the Sun continued to contract under its own gravity
until the pressure at the core grew to enormous proportions
(about 100 billion times the pressure of air on the Earth). A law of
nature states that if you squeeze anything it heats up. As the
centre of the Sun became more and more compressed the
temperature at the core reached about 15 million degrees
Celsius (27 million degrees Fahrenheit). This meant that the
protons (hydrogen "atoms") at the core possessed, in atomic
terms, huge amounts of energy. So much energy in fact, that
some of them could overcome any magnetic resistance and fuse
into deuterium, releasing even more energy in the process. This
is the first stage in the Sun's source of energy:
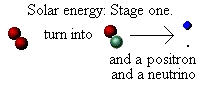
Further energy is provided by the positron. This is a form of
antimatter. It has exactly the same properties as an electron (in
terms of mass etc.), but has an opposite, and equally strong,
electrical charge. The Sun's core contains many "free" electrons
in what is called a "plasma" (a sort of high energy gas). The
positron soon meets an electron and when it does so the two
annihilate each other producing a high-energy photon, i.e. "light".
Our star is shining, but not yet in the visible part of the spectrum:

Helium-3
The second major stage is the formation of helium-3 (He-3),
again by a process of fusion. There are four isotopes of helium,
He-3, -4, -5, and -6. The latter two isotopes, He-5 and -6, have
short half-lives (in the case of He-5 only 6 x 10
–20
seconds!) and
we will not be concerned with them here. Each helium atom, by
definition, has two protons.
So far we have an "atom" of deuterium (a proton and neutron) in
a sea of highly energetic hydrogen "atoms" (protons). Sooner or
later (but usually sooner!) the two types of atoms will collide and
fuse. When they do so they combine to make the atomic nucleus
of helium-3, and eject yet another high-energy photon:
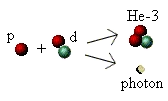
Helium-4
Lastly, the third stage is the production of helium-4. In this
process, two He-3 "atoms" come together and fuse, releasing
two protons. The two protons fly off in different directions and go
back to being hydrogen "atoms", from which they can take part
in the whole process again. The process looks like this:
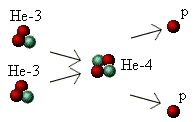
Throughout each stage some mass was converted into energy
and a total of 6 high-energy photons was produced. However,
we still haven't seen any visible light. The visible light from the
Sun is due to the ejected particles jostling with other atoms and
so transferring some energy to them. This causes the latter
atoms to emit photons with a wide range of frequencies,
including those in the visible part of the spectrum. At last, we
have visible sunlight!
The three stages outlined here are often taken together and
called the "proton-proton" chain.
The Total Energy
The total energy released in all three stages is around 4 x 10
–12
J (25 MeV). This is less than is produced during in a single
uranium-235 fission process. However, if we take the total
number of particles into account in each process, we find that we
get around 7 times more energy per particle in hydrogen fusion
than we get from uranium-235 fission. In other words, it is a
much more efficient process. Even so, only around 0.7% of the
mass of the Sun's protons eventually ends up as light.
The energies we have been talking about, while large on an
atomic scale, are still very small in everyday terms. However,
there is something we must take into account about the Sun, and
that is it's enormous! It has the same volume as 1.3 million planet
Earths. Some numbers about the rate at which nuclear fusion
takes place in the Sun will be instructive:

The amount of mass converted into energy at the Sun's core is
stunning. However, the Sun has a mass of around 2 x 10
30
kg
and has enough hydrogen to continue its proton-proton chain for
around another 4 billion years. After that, as we shall see, the
Sun will use another process to keep shining for a "little" longer.
Lastly, it's worth mentioning that the "sunlight" produced at the
core doesn't just fly off into space. As has been seen, the proton-
proton chain doesn't even produce light in the visible part of the
spectrum. Instead, the high-energy protons and resultant kinetic
energy produced induces other atoms in the Sun to vibrate and,
in turn, release photons of many different frequencies, including
those in the visible part of the spectrum. These photons are re-
absorbed and re-emitted by adjacent atoms, each, on average,
slightly closer to the surface of the Sun. Finally, after around 100
to 200 thousand years an atom at the surface of the Sun absorbs
and then re-emits a photon, which flies off into space. Then, if
heading in our direction, it takes around 8.5 minutes for the
photon to reach the Earth. All of the daylight that we use to see
by started on its journey a very long time ago.

(The dark blotches are sunspots – magnetic storms on
the Sun’s surface, many of them bigger than the Earth.)
The Sun: Turning mass into energy.

Fusion in other atoms - the Sun's End
Fusion is possible in atoms other than hydrogen and helium. In
fact, fusion, one way or another, is possible with almost any kind
of atom. In nature the heaviest atoms that we could encounter
are of uranium, but scientists have been able to "build" heavier
atoms in atomic accelerators, such as the one at CERN. This is
done by firing atoms at each other until they "stick" (fuse)
together. Most of the resultant atoms have very short half-lives.
Another feature of such fusion is that it takes a lot of energy
while little, or even none, is given back out.
It turns out that the atom that gives up the most of its mass in
the form of energy during fusion is the humble hydrogen atom,
i.e. the lightest atom of them all. As we progressively fuse
heavier and heavier atoms we get progressively less and less
energy back out. In fact, we get at least some energy back out
all the way up to the element containing 26 protons. That
element is iron (Fe). For iron, and all heavier atoms, we need to
put more energy in than we get back out in order to achieve
fusion. This is called an endothermic process.
As we have seen, the Sun's fusion is exothermic, that is, more
energy is "produced" than was entered into the system. We
have also seen that the Sun is slowly converting its hydrogen
into helium-4. Once the Sun has used up all of its hydrogen in
this process its core will contract because there is now not
enough radiation ("light" and so on) being produced to withstand
the gravitational pressure from the outer layers. Although the
core contracts, the very outer layers of the Sun will expand,
possibly out to the orbit of Mars (the Earth will be turned into a
wisp of gas in the process). The Sun will then be what is a called
a "red giant". The contraction of the core raises its pressure, and
by doing so also raises its temperature. This results in there
being enough energy for the He-4 to begin to fuse into (mostly)
carbon and oxygen, and so produces enough radiation energy
("light") to stabilise the star. Once the He-4 has been used up no
further fusion will take place at the core, and the Sun will
contract again into a state called a "white dwarf". At that point,
the Sun will no longer be active as such, and will just continue to
cool over many millions of years, eventually becoming a cold
"black dwarf":

Supernova
Apart from the fact that we live near it, there isn’t anything
particularly special about the Sun. It's just an average star.
Some stars are much heavier than the Sun, and these go
through further fusion chains, producing even heavier elements.
A detailed description of all the phases in a very massive star's
life is beyond the scope of this page, but we will look briefly at
what happens to a star with a mass greater than about four
times that of the Sun.
Such massive stars start by converting hydrogen into helium,
then, when the hydrogen is used up, helium into carbon and
oxygen, just as in the Sun. However, the core of such a star is
so massive that when the helium has been used up it
gravitationally contracts still further, and produces yet heavier
elements via the fusion process. These contractions continue
until the star starts to fuse its remaining elements into iron.
As we have noted, fusion into iron is endothermic, i.e. it takes
more energy to do than we get back out. For the star there is
now no radiation (of any significance) being produced to stop
the outer layers from gravitationally collapsing onto the core.
Within a few seconds of the star's core starting to fuse into iron,
the outer layers start accelerating inwards at tremendous
speeds. As they do so they become ever more dense as more
and more material is compressed together and accelerated. The
highly compressed material eventually reaches near-light speed
(in only a matter of seconds), before it crashes down onto the
dense core of the star. When this happens the core is
compressed slightly, then bounces back with such enormous
force that it blows the outer layers back out into space. The star
has, effectively, blown itself apart in what is called a supernova
explosion. The energy released in a single supernova is more
than many billions of atom bombs being detonated all at once.
The picture below shows the remnants of a supernova. This was
a "nearby" (168,000 light years away) supernova that was
recorded in 1987, and given the name SN1987A. The ring of
material that is expanding away from the central core is actually
a bubble of gas, but we are looking at it edge on:

The energy released in the explosion is such that many different
types of atoms in the expanding gas shell are fused together to
form all the elements heavier than iron; such as copper, gold
and uranium. Next time you see some gold try to remember that
it was created billions of years ago by a process of fusion during
the last moments of a massive star's active life!
Generally, what is left of the star is a rapidly expanding bubble
of gas rushing off into space, and a super-dense central core
composed almost entirely of neutrons. The pressure of the outer
layers crashing down onto the central core during the initial
implosion causes the atomic protons and free electrons in the
core to fuse into neutrons. The star is now called a neutron star.
From this point on, the bare core that the star has become starts
to cool, emitting energy into space as it does so.
For even more massive stars the end result is a black hole.
These stars have so much mass that as the outer layers hit the
core they compress it into such a state that even the resulting
neutrons are compressed into other particles, possibly, for a
very short time, the building blocks of neutrons and protons,
called quarks. This results in such an enormous mass being
squeezed down into such a very small size that, under the
pressure of its own gravity, the core continues to collapse until it
becomes "infinitely small". The gravitational pull of such an
object is so strong that not even light can move fast enough to
escape it, and we have a "black hole".

The Crab Nebula
The supernova that created the Crab Nebula was seen on Earth in
AD1054. What remains is an expanding gas cloud and a central
neutron star.

H-bombs
The process of hydrogen fusion that is the source of energy for
stars is the same as is used in H- (hydrogen) bombs. Briefly, in
an H-bomb, a small amount of hydrogen (usually the isotopes
deuterium and tritium) is compressed together to the point at
which it fuses into helium, releasing enormous amounts of
energy. It takes a huge amount of pressure to compress
hydrogen to the point at which it fuses, and in order to make
such a bomb light enough to be delivered by an aircraft or
missile, the hydrogen compressing "agent" is actually an atom
bomb surrounding the hydrogen.
Nuclear (atomic and hydrogen) weapons often have their
"yields" (explosive power) measured as the equivalent of the
energy released in a quantity of the explosive TNT, measured in
tons. An atom bomb's power is typically measured in kilotons,
i.e. thousands of tons. The two atomic bombs dropped on Japan
and the end of World War II were in the 15 to 20 kiloton range.
The more powerful H-bombs, however, have their yields
typically measured in megatons, i.e. millions of tons.
On detonation, an H-bomb produces He-3 and He-4 in the same
way as the Sun. While these two elements are harmless in
themselves, the atoms move at such high speeds that they can
damage anything around them. They can also strike other,
heavier, atoms, breaking them down into harmful radioactive
elements. And, of course, there is the radiation caused by the
triggering atom bomb. For these reasons, nuclear weapons
tests were eventually carried out under ground, where most of
the radiation could be contained. Even so, each detonation
caused environmental damage and still released a quantity of
harmful radiation from the atom bomb component.

Nuclear Power from Fusion
As described in another page in this series, nuclear power
stations use nuclear fission (the breaking apart of heavy
elements) as their source of power. This has a number of
disadvantages (but not any more than conventional power
stations, depending on your point of view). For example, using
fission as a power source produces radiation, and although it is
mostly contained there is the problem of both decommissioning
old nuclear power stations and the question of what to do with
the contaminated waste products. Allied to that, as with
conventional power stations, there is only so much of the raw
materials available from which to produce the power.
Great advances have been have made in using other,
renewable sources of power, such as energy derived from wind,
wave and solar sources. However, because of the limited total
renewable energy incident on the Earth, these sources of power
will never be enough to meet all of the world's rapidly growing
demands, and they are often expensive ways of producing
usable power in the first place. All in all, it would be good if we
could find a cheap, safe and abundant source of energy. So why
not use controlled fusion?
The answer is simple: we don't know how to, at least not yet.
Small scale fusion has been carried out for many years in
experimental situations, but controllable, large scale hydrogen
fusion has yet to be achieved.
There are three points to note about controlled hydrogen fusion:

The goal is to be able to find a way of making the process
practical and cheap. This is thought to be so important that
every major industrial nation on the planet has access to
accelerators, with the hope of finding a way of making the fusion
process work efficiently. There isn't even any guarantee that it
will work, but if it does we will have solved all of our energy
problems both now and a long way into the future. To this end a
number of countries are building experimental fusion reactors of
competing designs, often with much collaboration between
nations and scientists. However, the task is so difficult and
complex that even if successful it will still be decades before we
see the first commercial scale power plants come into operation.
If we do ever solve the problem of controlled nuclear fusion, at
its root will be the conversion of mass into energy, in
accordance with this little equation: